Fig.1: A causal correlation between more CO2 and warming has never been proven.
I first try to explain in simple language how the greenhouse (gh) effect works. It is important that everyone who has anything to do with this effect at least understands what is happening. This definition was found on the internet somewhere: “it is the capture of the sun’s heat in the lower atmosphere of a planet, because of the greater transparency of the atmosphere for visible radiation from the sun than for infrared radiation emitted from the surface of the planet”.
This definition is misleading because it focusses only on a small aspect of the gh effect. This is not the main reason why a planet with water such as Earth is 33K warmer in the lower regions than a planet without water. I show you schematically how the gh effect really works:
Sunlight (UV/IR) on water => water vapor => water vapor accumulates (sticks together due to strong VanderWaal forces) => cloud formations => there is movement of this moist air and clouds due to pressure- and temperature differentials => clouds and water vapor move to cooler areas => condensation takes place => heat of condensation is released in the air => this is equal to 2260 kJ per kg.
This is the main reason for the gh effect and it shows how heat spreads evenly all over the planet. Don’t forget this. That cold front coming up is really Mother Earth’s way to distribute the heat on our world to an average of about 14 or 15 degrees C. What the definitions in the textbooks want you to believe is that the main reason for the gh effect is that the gh gas slows or captures outgoing long wave radiation from Earth. That is also true, but it is only a very small aspect of the gh effect. Maybe just 5 or 10%?
You would have experienced this particular aspect for yourself in a few simple experiments: 1) You take a hot shower, turn off the tap and after ten minutes when you get out, you feel that the shower cabin is still warmer than the immediate temperature of the environment. The water vapor concentration that was relatively high and lingered in the cabin, retained heat and continued to radiate this, also to your body. 2) I remember that my mother mentioned that the weather was “sweltering” if it had been hot on a summer’s day and clouds rose late in the afternoon. The heat radiation from the ground then goes up to the clouds and returns again by the back radiation of the clouds. 3) On a completely cloudless day it happens that we reach for our sunglasses in the car while we drive away from the sun. So why is that? That is because, if the air moisture content is high, there can be back radiation from the water vapor in the spectrum of light and the wavelengths where we can ‘see’. Hence, 4) the colors of sunlight in the rain (rainbow) and the colors of a dewdrop. 5) This re-radiation or back radiation is also the principle of infra-red and UV & visible spectrophotometry. I remember that as a student I sometimes secretly lifted the cuvette holder a bit during a determination to see what happens when you turn the wavelength knob on the absorbing wavelength when measuring the standard solutions. You can then see that the path of light is somewhat obstructed. The light goes back, mostly in the direction of the source, but also a bit round. That is why in my days we used to talk about ‘extinction’. The light goes extinct at the absorbing wavelength. The transmission of the light or radiation through the molecule is then lower. In spectrophotometry, the degree of extinction at a certain wavelength is therefore an indication of the concentration of a certain component (Lambert-Beer).
We can conclude from all these simple experiments that in the wavelength ranges where absorption of photons has taken place, the individual molecule begins to behave like a very small spherical mirror. You can compare it with turning on your headlights in foggy weather: the light goes straight back to the source. The strength of this emission depends on the size of the amount of absorption that takes place in the molecule. If we assume that the molecule of the gas is a perfect sphere, 62.5% of a certain amount of light (radiation) is sent back in the direction it came from. The rest goes around.
We have already seen that both clouds and water vapor provide a clear greenhouse effect because there is reasonable mass for radiation to each other, for storing heat, and for condensing and releasing heat in colder places. Apart from clouds there is still a reasonable amount of water vapor (H2O) in the atmosphere, namely an average of approx. 1% v/v at normal sea level or approximately 0.4 or 0.5% on average over the entire atmosphere. See: Atmosphere of Earth – Wikipedia However, to take an average of the water vapor content over the entire atmosphere may actually not be appropriate here. Molecules of water vapor and water accumulate and are usually also held together by each other at lower altitudes due to the interactive forces specific only to water.
It is also important to remember that more than 99% of all gas in the atmosphere has almost no absorption and is therefore completely transparent for almost all wavelengths. Oxygen does have a tiny little bit of absorption at some very low wavelengths (see Fig.4 further down). So, apart from the greenhouse gasses, the entire atmosphere is actually transparent for all wave lengths. When our skin is exposed to sunshine, we become aware of the heat from the absorption of the radiation from the water just below our skin. Water has a number of absorptions and there is mass due to accumulation (vanderWaals) and therefore the radiation is converted into heat.
So, now we come to the so-called greenhouse effect by carbon dioxide (CO2). In this case we must first of all find that CO2 does not have any condensation taking place like that of water. So it does not directly release any energy up in the air. The second main point is that green house gasses like methane and CO2 react as an ideal gas in a vessel. That means that they spread equally in all directions due to diffusion. The molecules of these gases do not stick together. We therefore only have to look at the optical properties of such gas – i.e. what it does with the radiation that falls on it. Note that the amount of CO2 has increased from about 0.03% (315 ppmv) to 0.04% (420 ppmv) from about 50 years ago until now. The difference is indeed only 0.01% v/v. Compare this extra 0,01% now with about 50 times as much water vapor in the atmosphere and even more than 100 times as much water vapor at normal sea level. That is all excluding clouds. See Fig.2

Fig. 2: Water vapor up to ca. 3 km, where the gh effect is relevant, is still ca. 1 % v/v (10000 ppmv), 100 x more than the observed increase in CO2 over the past 50 years.
Now, carefully look at the Infra-Red spectrum of CO2

Fig. 3: The Infra-Red spectrum of carbon dioxide (CO2)
The more absorption, the lower is the transmission of radiation through the molecule, the more of that specific radiation is back radiated. The scale of transmission (y-axis) is sometimes multiplied by 100x to indicate the percentage of transmission through a gas or liquid.
The spectrum shows that, indeed, CO2 absorbs strongly in the region 14-15 um where earth also emits strongly. Remember that every CO2 molecule that has been added to the atmosphere is actually surrounded by hundreds of other molecules that are mostly completely transparent to the back radiation / emission by the CO2. So what happens is that the rising radiation of 14-15 um from the earth does bump into the CO2 molecules, but then it is radiated back, as mentioned, approx. 62.5% in the direction of the source. That means that that radiation is partially coming back to earth, where it can cause some warming if it eventually comes into contact with water/water vapor/clouds that has mass (water also has absorption at 14-15 um) or perhaps comes into contact with some other molecules of CO2 or water vapor that have not yet absorbed at 15 um.
At this point in time the question arises: But what about the other absorptions of CO2 such as those at 4.3um and 2.3um that are clearly in the spectrum of the sun, not that of earth. There are also other absorptions of CO2 that were not visible with the measurement method used in Fig. 3, namely those in the nearby infra-red. Fig. 4 shows the spectrum of earth’s light in the nearby IR, as captured or displayed with instruments on Earth via the dark side of the moon. Source here
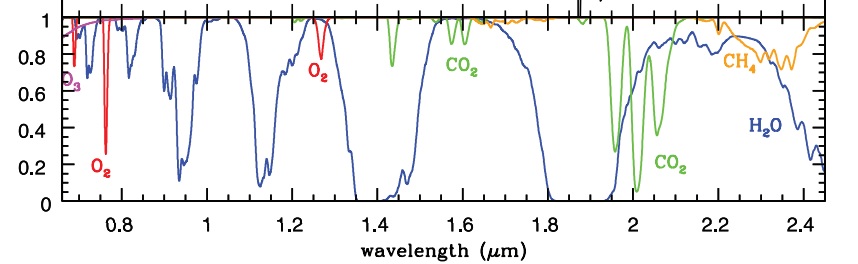
Fig. 4: Re-radiation from greenhouse gases (no clouds) in the nearby Infra-Red
(via the moon back to earth – green line is back radiation by CO2)
We now see that light from the sun that fell on a CO2 molecule e.g. around 2um, was absorbed, and then reflected into space by the molecule (green line, Fig. 4). We can measure this emission via the reflection by the moon. Schematically, what is represented by Fig.4:
Sunlight to the earth = > absorption of radiation by CO2 = > radiation back to the source and to space = > therefore also to the moon = > via the dark side of the moon back to the earth = > to the measuring instrument.
The problem of a possible greenhouse effect due to CO2 now lies at the point that due to the relevant gas laws there can be no accumulation of that gas in the lower atmosphere, and subsequent condensation as is the case with water (clouds) and water vapor. CO2 is ca. 410 parts per million, man is claimed to be responsible for 100 ppm since the start of the industrial age. Even that claim is debatable as due to Henry’s Law there is also extra CO2 outgassing due to the increasing temperature of the oceans. Never mind that, just remember that every added CO2 molecule is surrounded by ca. 10,000 other molecules in the atmosphere. I am saying that there is no mass for heat absorption except by the single molecule CO2 itself amidst 10000 other molecules after which it starts emission.
As a result, we can simply evaluate the effect of more CO2 directly by calculating the energy of the radiation of CO2 back to the earth and comparing this with the energy of the back radiation by CO2 to the sun and space.
And here’s my moment to open the champagne. The electromagnetic radiation from earth is generally misrepresented as a ‘blackbody’ type of distribution that starts at 4 um (Wikipedia/ KNMI). But something is not right there. Here is the state of affairs as presented in a textbook by Petty and apparently relies on actual measurements that have been carried out. The source is indicated in the description below the graph.

Fig. 5 = Fig. 8.2 (above)
What does all of this mean?
This being the case, we can actually do a simple calculation over all wavelengths of Fig. 3 + Fig. 4. All radiation to earth below 22 um relevant to CO2 comes from the sun for 12 hours a day, on average. The radiation from earth runs upwards from 6 um to 22 um for 24 hours a day. The amount of energy caused by the obstruction of radiation by CO2, both by the radiation of sun and earth radiation, is compared with each other. The amount of energy per blocked wavelength over the entire spectrum of CO2 can be calculated piece-by-piece with Wien’s Law here. Each piece is also multiplied by the transmittance between 0 and 1. Because: the lower the transmission, the greater the obstruction by the CO2 molecule. I’ve done all that here:
Summary of analysis CO2 spectrum NIST (1)
The results of my calculations are in the first three rows of columns K, L and M. Note that the total cooling effect (by the back radiation from CO2 to the sun and space) was divided by two, since the sun shines on average for 12 hours per day while earth shines for 24 hours a day.
CONCLUSION
Just as I have always suspected, the result is negative: there is in fact no warming due to the addition of more CO2 in the atmosphere. Below is a list of studies by people who have investigated same problem in different ways and somehow have come to the same conclusion.
John O’Sullivan (HT: Alan Siddons) 2016
- Seim & Olsen 2020
- Rasool and Schneider (see Science 173, 1971 138) https://www.science.org/doi/abs/10.1126/science.173.3992.138
- Joseph Reynen 2022 https://principia-scientific.com/wp-content/uploads/2023/02/SaturationIIIupdated.pdf
- Ronald D. Voisin 2022
An Engineer’s Climate Theory – January_ 2022.pdf(Shared) – Adobe cloud storage
6. Roger Ian Holmes
https://sciencepublishinggroup.com/journal/paperinfo?journalid=161&doi=10.11648/j.earth.20170606.18


The results as reported were not surprising, given the results of my statistical analyses. See: https://breadonthewater.co.za/2021/11/25/an-inconvenient-truth/
My evaluation does not mean that there might still be some indirect warming effect, caused by CO2, locally, due to the increase in greening which changes earth’s albedo.
https://breadonthewater.co.za/2022/01/10/global-warming-due-to-ehhh-global-greening/
Please feel free to use any or all the information given in this post for your own blog or project. However, if you do, it would be nice if you would consider making a donation to our charity, Heart for Children. We give financial help to foster- and safe homes.
For more information, see here: https://heartforchildren.co.za/
I believe Wikipedia may have the percentage of water vapor in the atmosphere wrong. many other sources claim from a trace to 6% depending on temperature and humidity
David,
I knew about the 1% water vapor at sea level (or below 1 km as shown in Fig.2) for a long time which is why I came to investigate why anyone would be worried about d[CO2] of only 0.01%. The main thing about my report here is that I find that the nett effect of more [CO2] does not cause any direct warming of the atmosphere. As I said, there may be an indirect effect by causing more greenery, which may slightly change earth’s albedo.
Henry,
It is much easier to understand how greenhouse effect works.
Here greenhouse effect brief description : CO2 absorb some IR energy and then re-emit it according to black body radiation. Half of that energy returned back to surface. The accepted value of this radiative forcing is about 3 W/M2.
Now almost same description (try to prove me wrong) : thin layer of atmosphere (even without greenhouse gases) has approximately same temperature as the surface of Earth due to direct contact and convection. Such layer emit energy according to black body radiation. Half of this energy returned back to surface. Radiative forcing will be in the range of 200 W/M2. Where is this energy?
Nikolai
Now you say:
The accepted value of this radiative forcing is about 3 W/M2.
Sorry. The ‘calculated’ value is actually 3.7 W/m2 for a doubling of CO2.
The increase in CO2 is about 2 ppmv per year, calculated from about 50 years ago. The increase was therefore 100/320 (1972) = approx. 30% So it may take another 100 years before we have a doubling, calculated from 1972.
3.7/150 = 0.025 W/m2
CO2 is therefore ‘calculated’ as a factor more than 10 times smaller than what they measure in the Netherlands in terms of the increase in radiation (because of fewer clouds and cleaner air)
However, according to my calculations as shown in this post, the effect of 3.7W/m2 due to a doubling of more CO2 is not even there AT ALL.
Keep up the good work Henry. I no longer post on wuwt due to the registration process. Meanwhile I’ve put around 7 tons of good, dry hardwood up the chimney so far this winter in an attempt to warm up the atm…but for some reason it’s still cold outside but it does stay warm in the house…thanks to IR radiation!
Last we corresponded was the scamdemic I think…did the alleged dip in CO2 emissions due to the lock down cause any measurable impact on anything? I assume not or we’d be hearing about it on the MSM?
And your favorite topic of UV…I have a woke sibling with a PhD…she watched a show about deep diving and how that color appearance changes with depth…
First reds are lost, then orange, yellows, and so on…I tried to explain to her that UV flux of the sun changes over time resulting in changes in energy absorption of the ocean which in turn influences climate over (lag) times…and that this process has more impact that the mythic CO2…she almost got it I think…but it took the tv show and the divers suit going from red to black to get her noodle working…but…she may have got it!!
True. The variation in UV from the sun probably determines most of the climate.
Indeed, firing up some wood helps greening the earth.
Nice. Referred here from WUWT. This takes me right back to Organic Chemistry, second year. The peaks are the bottoms and the total absorption is the area below the top. H2O has way more absorption than CO2 on this graph–which would not take into account that water vapor is roughly 1 to 5% of the atmosphere, while CO2 is .042% currently. That’s at least 20 times as much. The CO2 might have a net warming effect by slowing the radiation of heat out from the Earth, but it would not be detectable against 20 times as much of a molecule that absorbs about 9 or 10 times more energy (my eyeball estimate).
True. Is what I am also saying. There is no mass as there is no accumulation. \ And going solely on back radiation, the net effect is zero as it back-radiates as much energy to space as to the earth.
Note that I do not say that there is no indirect warming effect of more CO2. I have found in several of my studies reported elsewhere, that the increase in CO2 in the atmosphere leads to more greening. In its turn, this leads to higher minima as the reaction at night with CO2 to form leaves, wood and fruits, is exothermic. The greening of earth also changes albedo. Looking from the outside in, this makes it somewhat blacker, which also attracts heat. The question now is what do you want: Do you want greener + warmth or do you want less green + cold? Ironically, identical findings as mine on this were already proposed after a study by John R. Christy (et al) back in 2006:
https://journals.ametsoc.org/view/journals/clim/19/4/jcli3627.1.xml
Well worth a re-read since you posted on WUWT’s recent CO2 saturation article with 100’s of comments. We’ve had blocks of days here in East TN this month where the temperature has not risen above freezing. This was unusual even back in the 60’s and 70’s…mild summer, pleasant fall…and now run away cooling…coincidence? I think not…it can only be that you’re right Henry…it’s the CO2 cooling effect!!! Meanwhile, the fireplace keeps us warm even when the more complex technology fails….