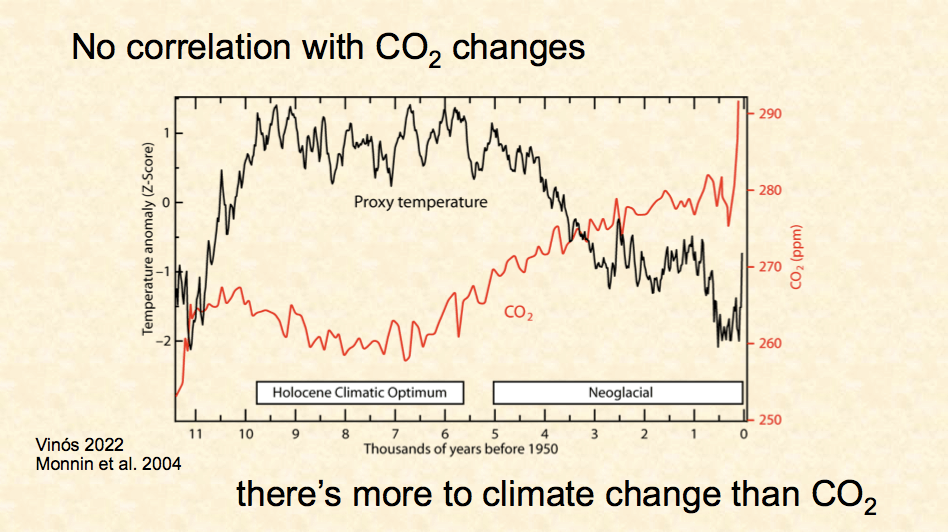
Fig.1: A causal correlation between more CO2 and warming has never been proven.
I first try to explain in simple language how the greenhouse (gh) effect works. It is important that everyone who has anything to do with this effect at least understands what is happening. This definition was found on the internet somewhere: “it is the capture of the sun’s heat in the lower atmosphere of a planet, because of the greater transparency of the atmosphere for visible radiation from the sun than for infrared radiation emitted from the surface of the planet”.
This definition is misleading because it focusses only on a small aspect of the gh effect. This is not the main reason why a planet with water such as Earth is 33K warmer in the lower regions than a planet without water. I show you schematically how the gh effect really works:
Sunlight (UV/IR) on water => water vapor => water vapor accumulates (sticks together due to strong VanderWaal forces) => cloud formations => there is movement of this moist air and clouds due to pressure- and temperature differentials => clouds and water vapor move to cooler areas => condensation takes place => heat of condensation is released in the air => this is equal to 2260 kJ per kg.
This is the main reason for the gh effect and it shows how heat spreads evenly all over the planet. Don’t forget this. That cold front coming up is really Mother Earth’s way to distribute the heat on our world to an average of about 14 or 15 degrees C. What the definitions in the textbooks want you to believe is that the main reason for the gh effect is that the gh gas slows or captures outgoing long wave radiation from Earth. That is also true, but it is only a very small aspect of the gh effect. Maybe just 5 or 10%?
You would have experienced this particular aspect for yourself in a few simple experiments: 1) You take a hot shower, turn off the tap and after ten minutes when you get out, you feel that the shower cabin is still warmer than the immediate temperature of the environment. The water vapor concentration that was relatively high and lingered in the cabin, retained heat and continued to radiate this, also to your body. 2) I remember that my mother mentioned that the weather was “sweltering” if it had been hot on a summer’s day and clouds rose late in the afternoon. The heat radiation from the ground then goes up to the clouds and returns again by the back radiation of the clouds. 3) On a completely cloudless day it happens that we reach for our sunglasses in the car while we drive away from the sun. So why is that? That is because, if the air moisture content is high, there can be back radiation from the water vapor in the spectrum of light and the wavelengths where we can ‘see’. Hence, 4) the colors of sunlight in the rain (rainbow) and the colors of a dewdrop. 5) This re-radiation or back radiation is also the principle of infra-red and UV & visible spectrophotometry. I remember that as a student I sometimes secretly lifted the cuvette holder a bit during a determination to see what happens when you turn the wavelength knob on the absorbing wavelength when measuring the standard solutions. You can then see that the path of light is somewhat obstructed. The light goes back, mostly in the direction of the source, but also a bit round. That is why in my days we used to talk about ‘extinction’. The light goes extinct at the absorbing wavelength. The transmission of the light or radiation through the molecule is then lower. In spectrophotometry, the degree of extinction at a certain wavelength is therefore an indication of the concentration of a certain component (Lambert-Beer).
We can conclude from all these simple experiments that in the wavelength ranges where absorption of photons has taken place, the individual molecule begins to behave like a very small spherical mirror. You can compare it with turning on your headlights in foggy weather: the light goes straight back to the source. The strength of this emission depends on the size of the amount of absorption that takes place in the molecule. If we assume that the molecule of the gas is a perfect sphere, 62.5% of a certain amount of light (radiation) is sent back in the direction it came from. The rest goes around.
We have already seen that both clouds and water vapor provide a clear greenhouse effect because there is reasonable mass for radiation to each other, for storing heat, and for condensing and releasing heat in colder places. Apart from clouds there is still a reasonable amount of water vapor (H2O) in the atmosphere, namely an average of approx. 1% v/v at normal sea level or approximately 0.4 or 0.5% on average over the entire atmosphere. See: Atmosphere of Earth – Wikipedia However, to take an average of the water vapor content over the entire atmosphere may actually not be appropriate here. Molecules of water vapor and water accumulate and are usually also held together by each other at lower altitudes due to the interactive forces specific only to water.
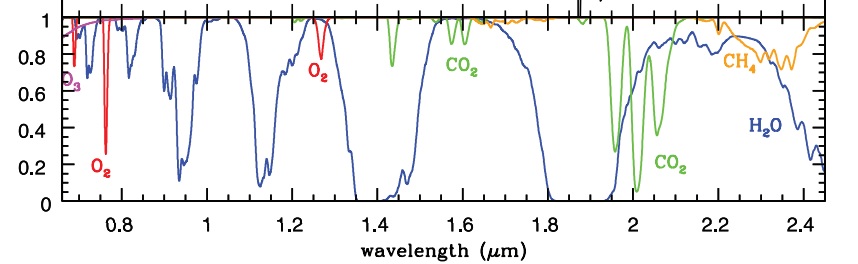
It is also important to remember that more than 99% of all gas in the atmosphere has almost no absorption and is therefore completely transparent for almost all wavelengths. Oxygen does have a tiny little bit of absorption at some very low wavelengths (see Fig.4 further down). So, apart from the greenhouse gasses, the entire atmosphere is actually transparent for all wave lengths. When our skin is exposed to sunshine, we become aware of the heat from the absorption of the radiation from the water just below our skin. Water has a number of absorptions and there is mass due to accumulation (vanderWaals) and therefore the radiation is converted into heat.
So, now we come to the so-called greenhouse effect by carbon dioxide (CO2). In this case we must first of all find that CO2 does not have any condensation taking place like that of water. So it does not directly release any energy up in the air. The second main point is that green house gasses like methane and CO2 react as an ideal gas in a vessel. That means that they spread equally in all directions due to diffusion. The molecules of these gases do not stick together. We therefore only have to look at the optical properties of such gas – i.e. what it does with the radiation that falls on it. Note that the amount of CO2 has increased from about 0.03% (315 ppmv) to 0.04% (420 ppmv) from about 50 years ago until now. The difference is indeed only 0.01% v/v. Compare this extra 0,01% now with about 50 times as much water vapor in the atmosphere and even more than 100 times as much water vapor at normal sea level. That is all excluding clouds. See Fig.2
















Recent Comments